Introduction
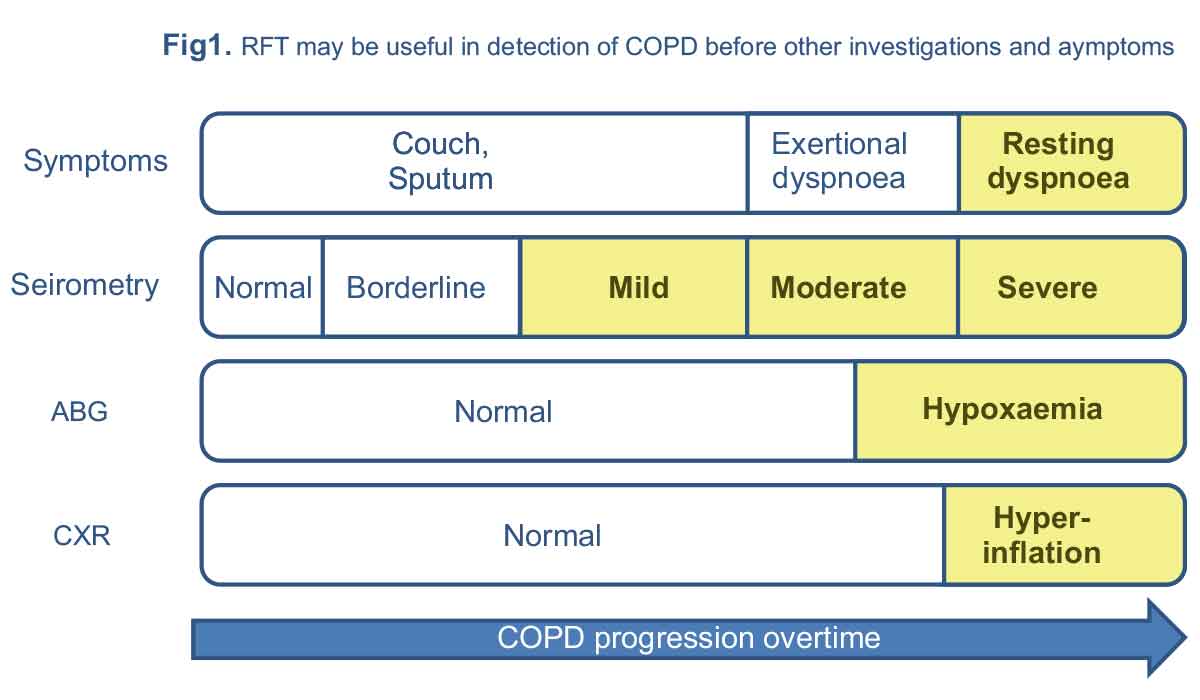
Although respiratory function tests are an integral part of the diagnosis and management of respiratory diseases, they are not diagnostic for any given disease. Respiratory function tests may be able to identify and quantify respiratory system functional abnormalities years before other investigations become abnormal or patients become worried about their symptoms (Figure 1).
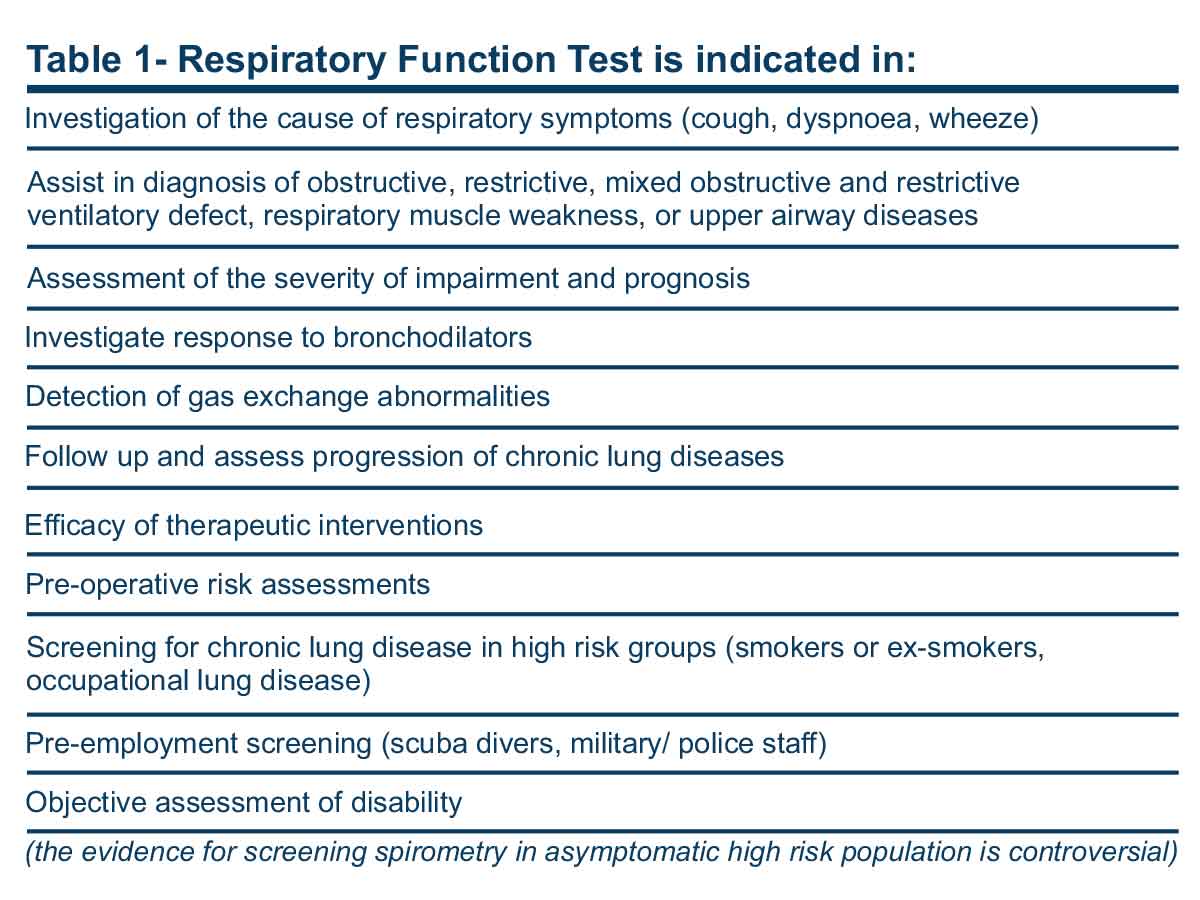
Despite their ability to answer many clinically relevant questions (Table 1), they are underused to a great extent. There are patients who have had invasive cardiac tests to assess their symptoms before a respiratory function test reveals the real cause of their complaint. Lack of confidence in the interpretation of respiratory function tests may be one of the reasons for this. The purpose of this article is to improve general practitioners’ abilities to identify the indications and interpretation of these tests.
Respiratory Function Test
Respiratory function tests may have different parts, including spirometry, diffusing capacity, measurement of lung volumes, tests of respiratory muscle strength, and bronchial provocation tests. Spirometry is used to measure dynamic lung volumes, body plethysmography to measure static lung volumes, and diffusion capacity to measure gas transfer abilities of the lung.
Lung Volumes and Capacities
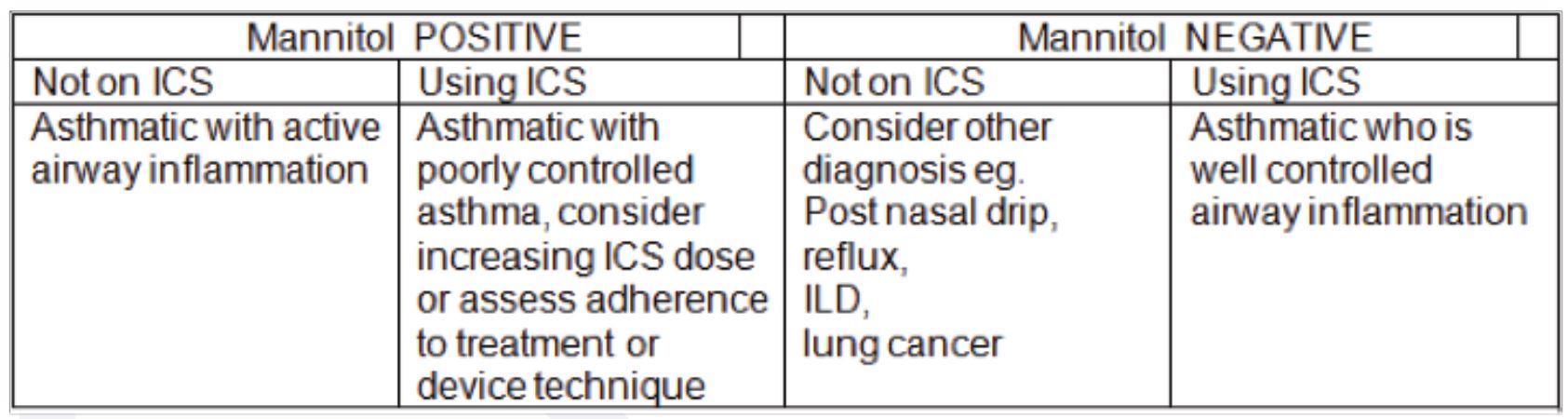
Figure 2 shows static lung volumes and capacities. The summation of two or more volumes is called capacity.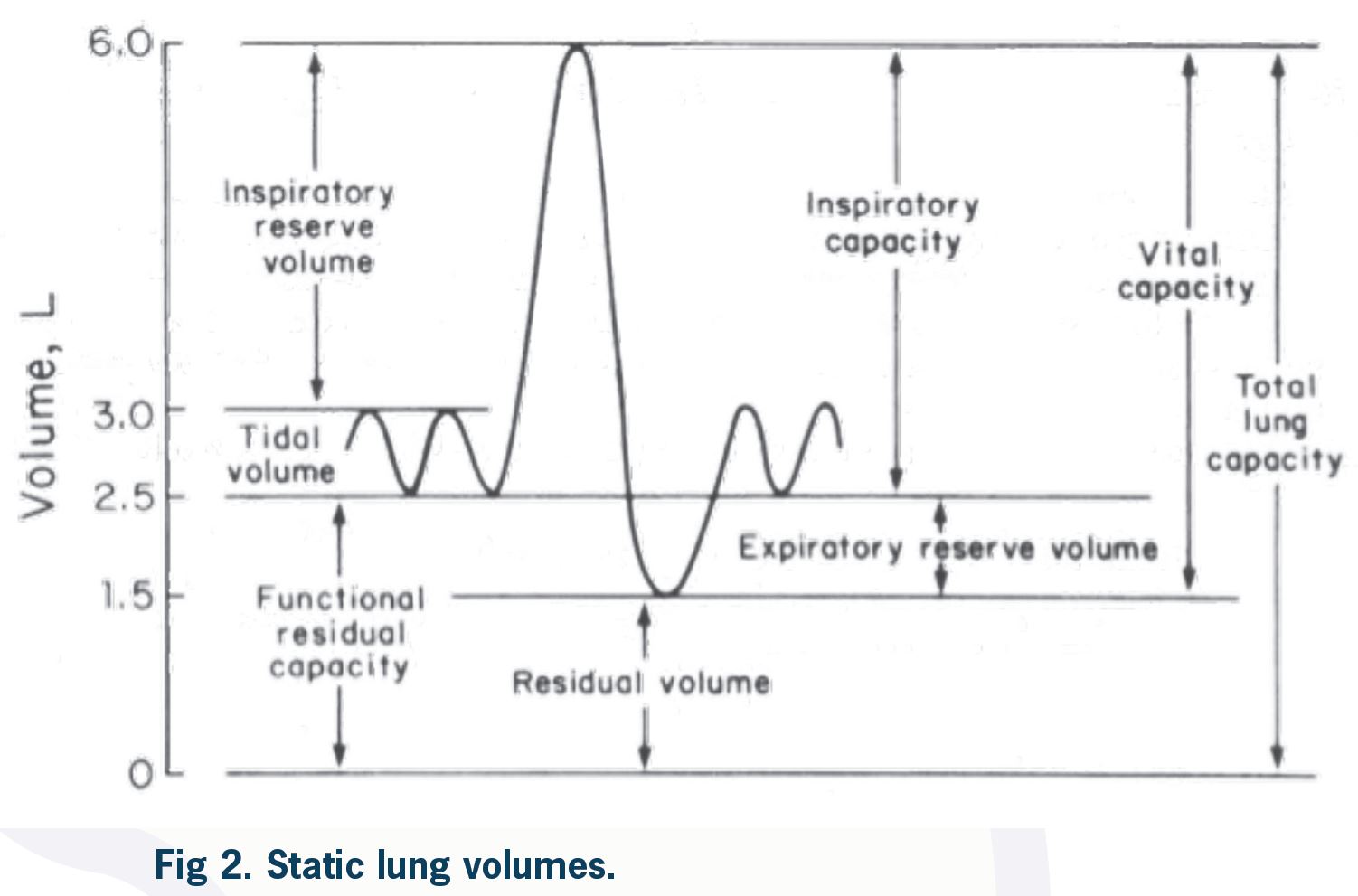
Spirometry
Testing
Spirometry is a tool to assess ventilatory function by measurement of inspired and expired volumes over time (i.e., dynamic lung volumes). A subject is asked to inspire maximally and then exhale forcefully and completely into a spirometer.
Volume Measurements
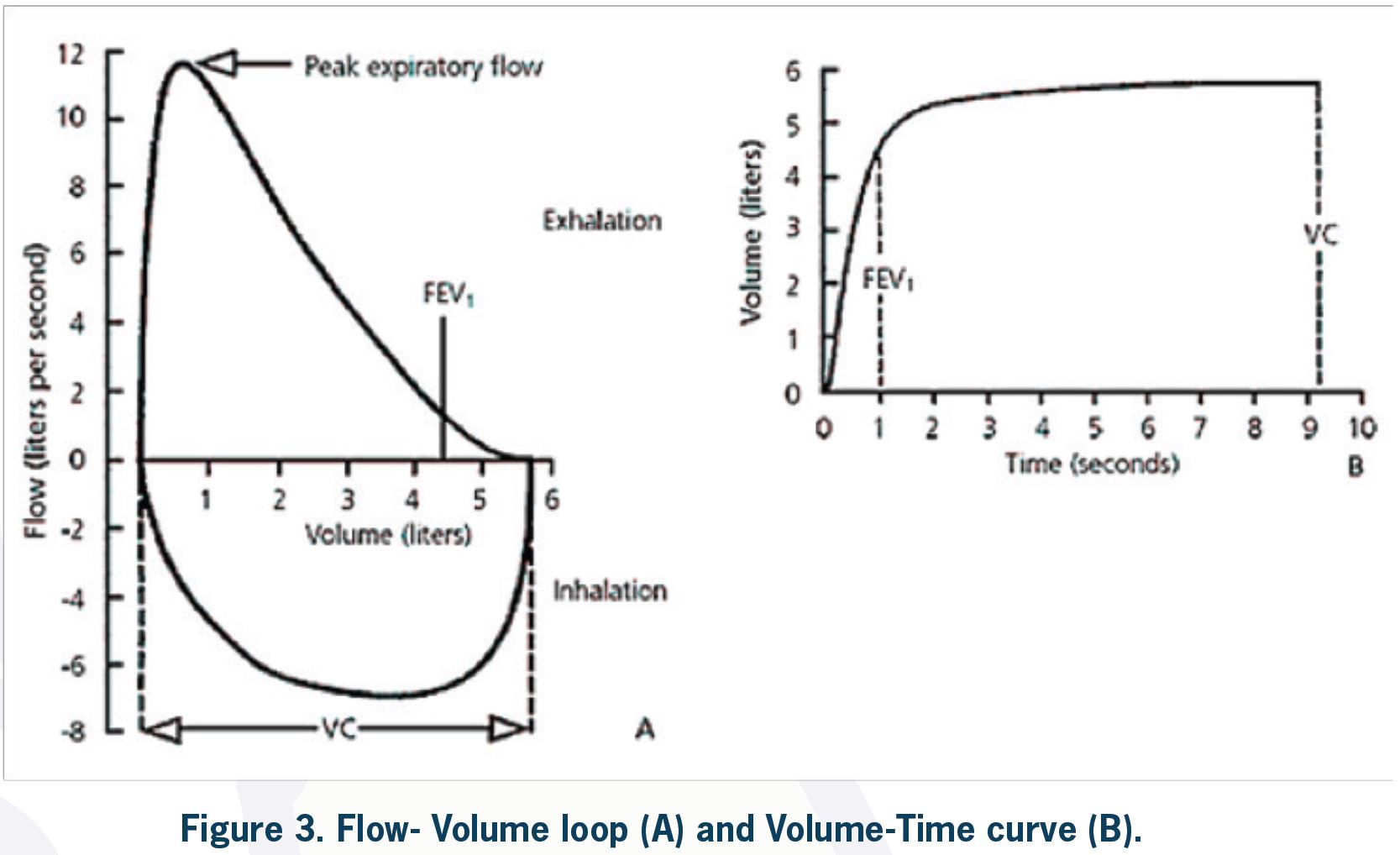
The volume of air exhaled in the first second of forceful expiration (forced expired volume in one second = FEV1) and the total volume exhaled forcefully after maximal inspiration (the forced vital capacity = FVC) are recorded, and the FEV1:FVC ratio (forced expiratory ratio = FER) is calculated.
Flow Measurements
During the FVC maneuver, peak expiratory flow (PEF), which is the maximum flow generated during forceful exhalation, may also be measured. PEFR (peak expiratory flow rate) is reliable and easy to measure by the patient at home and can be valuable in asthma management. The average of expiratory flow over the middle 50% of forced expiration is called FEF25%-75%. This value, compared to volume measures (i.e., FEV1), may be considered a more sensitive measure of small airways disease to detect early obstruction in COPD and asthma. Because flow measurements are more dependent on patient effort compared to volume measurements (FEV1 and FVC), they are highly variable and not specific for small airways disease.
Baseline Values
The baseline values for each patient are determined by patient age, sex, height, and ethnicity. After entering these parameters into the device, the baseline (normal) predicted values for a particular patient are calculated, and the results of the patient’s test are compared with these normal values and presented as percentages and raw numbers.
The baseline values have been well validated in population studies for Caucasians. In non-Caucasian populations, a correction factor is used. These baseline values are validated for patients between 8–80 years of age, and values beyond these age groups are extrapolated. This may cause some degree of inaccuracy in the interpretation of lung function tests in these age groups.
Test Performance
It is very important to direct the patient to perform the test properly. The patient must reach maximum inspiration before rapid expiration starts. Expiration is continued with maximum effort until the patient cannot exhale any more air. There are criteria for the start and end of the test. For an acceptable performance, the reproducibility criteria must be met, and a minimum of 3 acceptable attempts is required. Artefacts may also occur during the test, which can make interpretation difficult. These artefacts include cough, glottis closure, submaximal effort, air leak, and obstructed mouthpiece. Reproducibility of the tests and the presence of artefacts are assessed by the technician. These are evaluated while the patient is performing the test and also by observation of different flow-volume loops or volume-time curves. Poor test performance may resemble disease patterns and cause misinterpretation. For example, FVC is underestimated after a submaximal effort, and spirometry may be suggestive of a restrictive ventilatory defect or an obstructive defect may be missed (lower FVC causes a higher FEV1/FVC ratio).
Interpretation
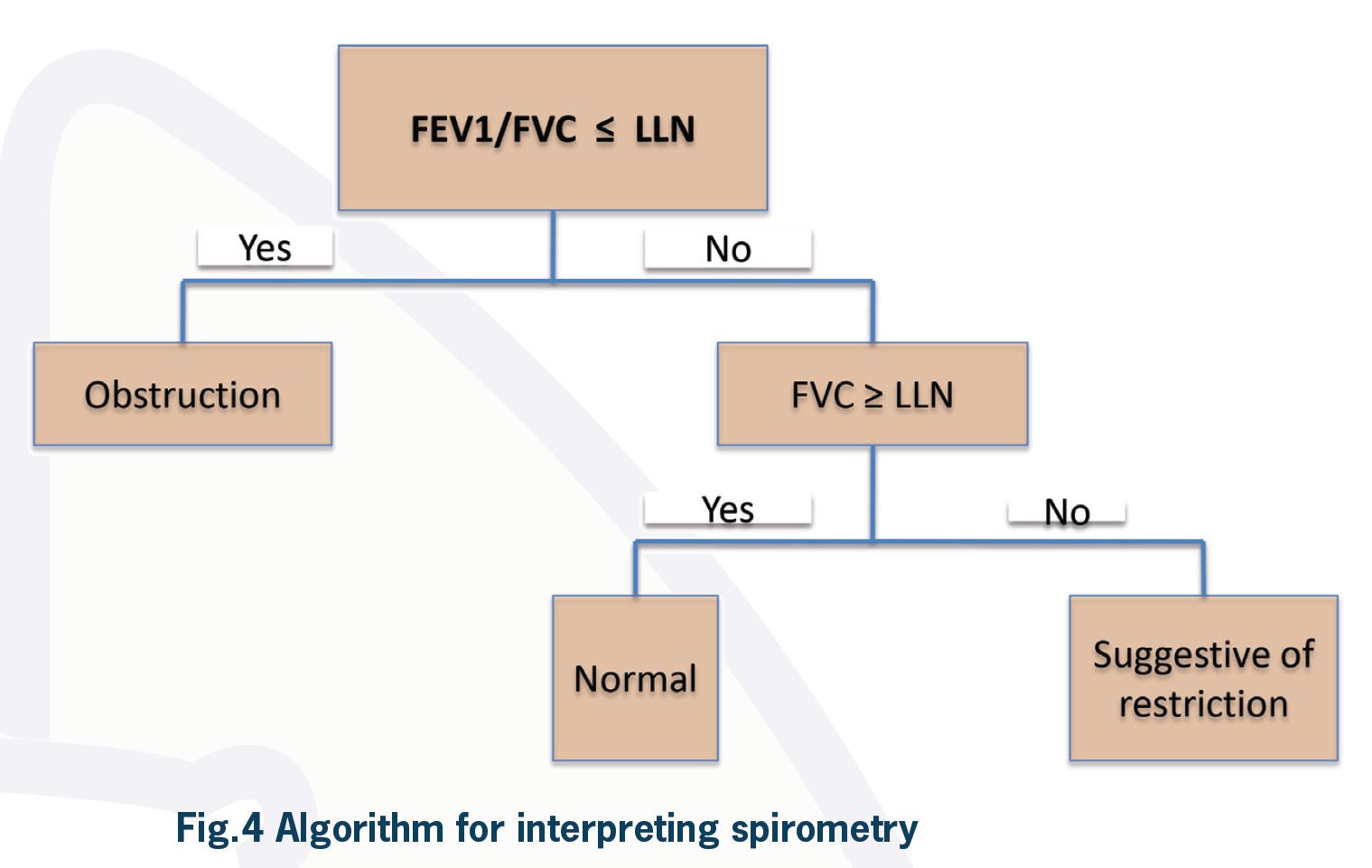
An approach to the interpretation of spirometry is illustrated in Figure 4. The first step is to look at the FEV1:FVC ratio (FER) and compare it to the predicted value for the patient. If FER is less than the lower limit of normal for the patient, there is an obstructive ventilatory defect. However, in other classification systems, specifically for research purposes, airflow obstruction is defined by FER less than 70%. If FER is more than the lower limit of normal, the patient does not have an obstructive defect.
If FER is not reduced to less than what is predicted for the patient, then an obstructive ventilatory defect is ruled out. This result can be seen in a normal patient or patients with restrictive defects. Looking at vital capacity (VC) or FVC is the next step to differentiate between these two. If FVC or VC is less than the lower limit of normal for a particular patient and FER is normal, spirometry is suggestive of a restrictive ventilatory defect. This finding has to be confirmed by measurement of total lung capacity, which is the gold standard for the diagnosis of restriction. Normal VC or FVC rules out a restrictive defect.
Reduced FVC when FER is reduced most likely indicates hyperinflation as a result of severe airflow obstruction. It may also be due to a mixed ventilatory defect. Measurement of lung volumes is necessary to differentiate between these. A mixed obstructive and restrictive ventilatory defect is present when both FER and TLC are reduced.
There are patients who do not follow the above algorithm. Normal spirometry does not exclude severe lung disease. For example, in a subgroup of patients with combined emphysema and interstitial lung disease, there is a relative preservation of lung volumes with normal spirometry and a marked reduction in DLCO. However, this group of patients will have severe limitations in gas transfer properties, which will be discussed later in this article.
After one is diagnosed with airflow obstruction (FER < lower limit of normal), the next step is to classify the severity of the obstructive defect and to assess for the presence of a bronchodilator response (suggestive of current asthma).
Severity of Limitation
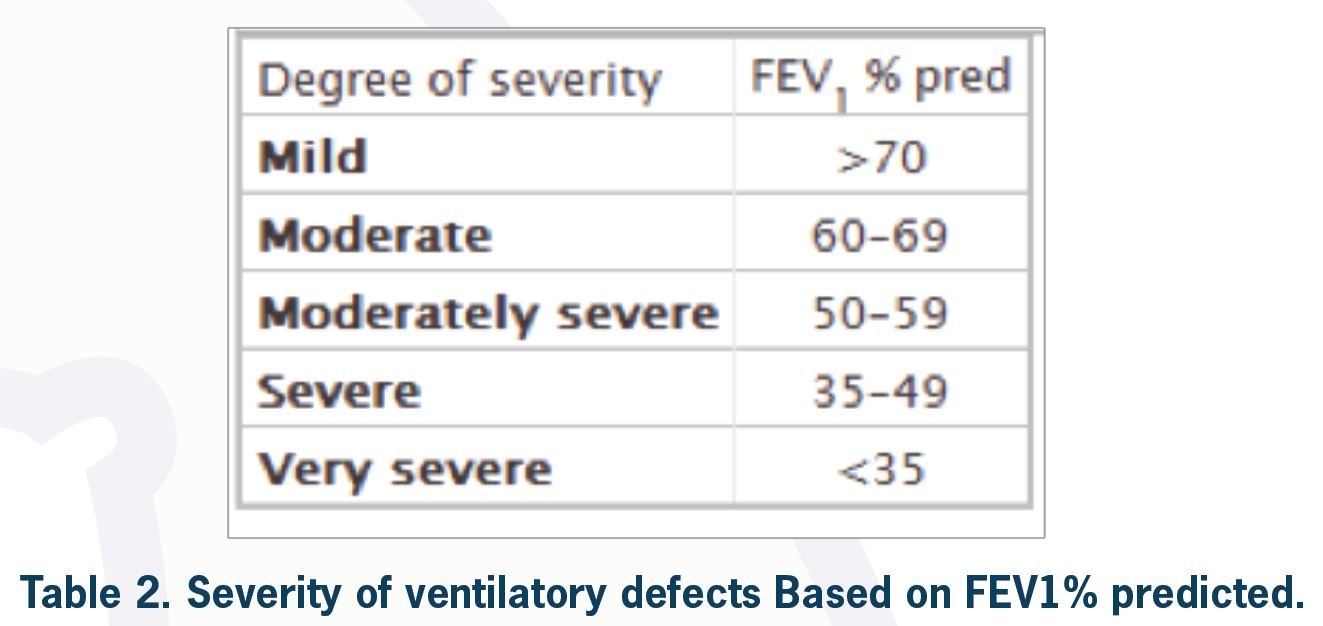
To classify the severity of a ventilatory defect, one needs to look at FEV1% predicted for the patient. According to the ATS/ERS task force, the degree of severity is as shown in Table 2.
As an example, if a patient has FER < predicted LLN and FEV1 = 100% predicted, by definition, this patient has a mild obstructive ventilatory defect (although this can be considered a normal variant depending on clinical information). However, this can sometimes be considered normal, especially in young populations.
Bronchodilator Response
Spirometry can be performed before and after the administration of an inhaled bronchodilator to test for a significant response, which is suggestive of reversible airflow obstruction. To assess bronchodilator response, the patient is given 400 micrograms of inhaled salbutamol (four separate puffs with 30-second intervals) via a spacer, and spirometry is repeated after 15 minutes. If FEV1 and/or FVC is improved by more than 200 milliliters and 12% after salbutamol use, the bronchodilator response is positive. This is suggestive of bronchial hyper-reactivity. In an appropriate setting, this result can be consistent with uncontrolled asthma. Bronchial hyper-responsiveness has clinical implications. It may suggest benefit from further treatment with inhaled corticosteroids or the addition of long-acting bronchodilators.
If the clinician is looking for reversibility in lung function to investigate their patient for possible asthma, the patients should not take their short-acting bronchodilator 4–6 hours before spirometry and long-acting bronchodilator 12–24 hours prior to testing.
Review of Flow Volume Loop (FVL)
Some practitioners review the flow volume loop even before looking at the results of spirometry. FVL can give critical information regarding patient performance and can also show characteristic features for lesions in the trachea or upper airway.
Pathologic FVLs
As mentioned previously, useful information regarding test performance and artefacts during spirometry can be obtained by looking at FVL. Flow volume loops are also valuable in diagnosing upper airway (intrathoracic or extrathoracic) pathologies.
Diffusing Capacity
Carbon monoxide is the gas used to measure the diffusing capacity of the lung (“window on the pulmonary microcirculation”). Because of its high affinity to hemoglobin, carbon monoxide is considered a diffusion-limited gas. Nitrogen and oxygen are considered perfusion-limited and both perfusion/diffusion-limited gases retrospectively.
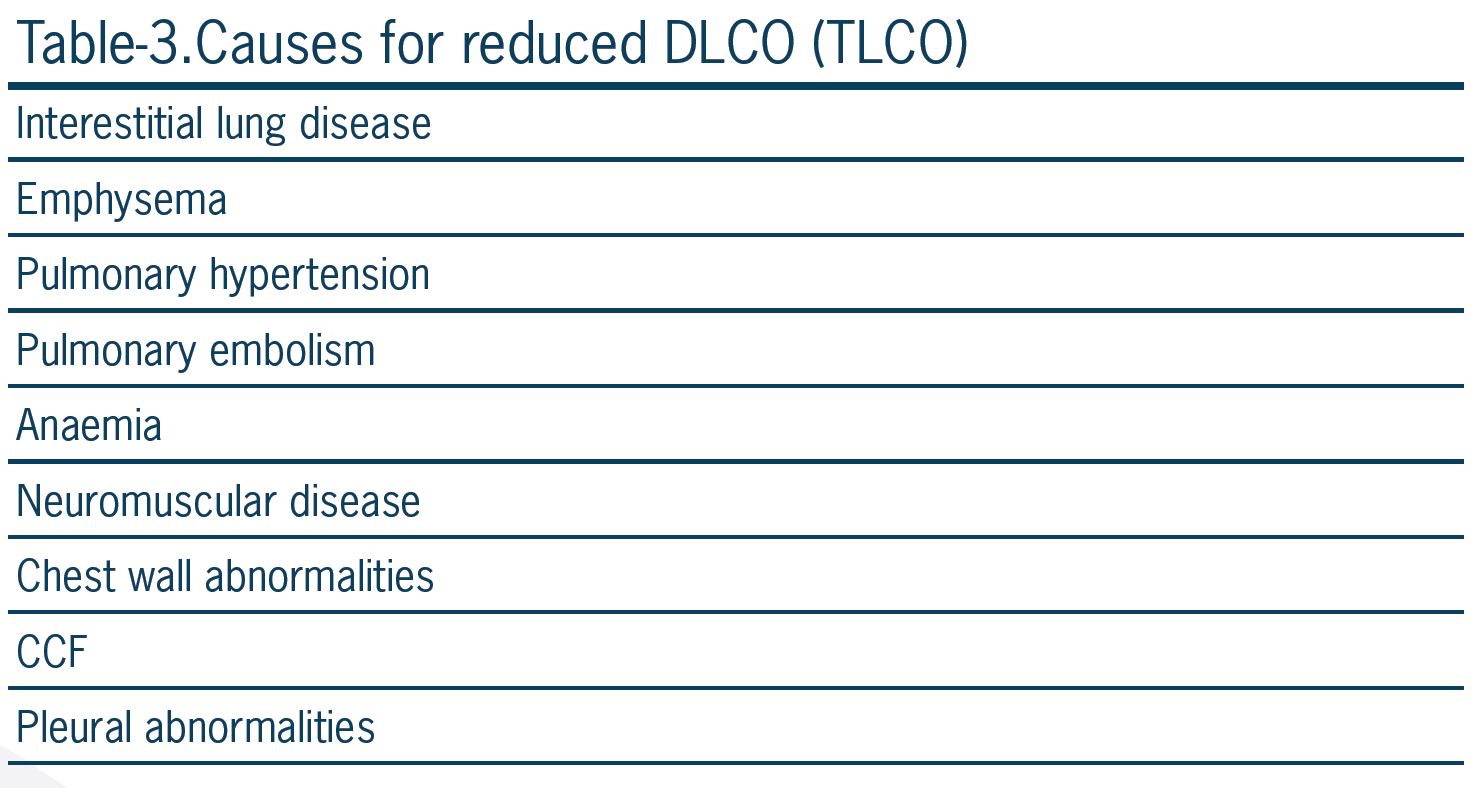
Carbon monoxide has 200 times more binding affinity to hemoglobin compared to oxygen. This property of carbon monoxide prevents a high concentration of the gas in the capillary blood, and tension across membranes does not occur. So gas concentration is not usually a limiting factor for its transfer across the alveolar-capillary membrane. The causes of limitation in diffusing capacity for carbon monoxide (DLCO) are listed in Table 3.
(TLCO = Transfer factor for carbon monoxide) Similar to normal values for spirometry, DLCO predicted values are determined by the patient’s sex, age, height, ethnicity, and altitude (inspired oxygen concentration). There are many other factors that can affect DLCO, including circadian rhythm, menstrual cycle, smoking, bronchodilator use, exercise, hemoglobin concentration, carboxyhemoglobin concentration, body position, and obesity in women (due to overestimation of the normal value for DLCO in obese women).
In many respiratory laboratories, hemoglobin concentration is measured by a finger prick test, and measured DLCO is corrected for hemoglobin. Clinicians always look for corrected DLCO to assess diffusion limitation.
It is also important to correct DLCO for alveolar (lung) volume (VA). In most cases with reduced lung volume, DLCO is also reduced. It is not clear whether the reduction in diffusion capacity is due to extrathoracic causes (including chest wall and pleural abnormality, neuromuscular disease, or poor test performance) or a real reduction in diffusion capacity.
If DLCO corrected for alveolar volume (KCO = DLCO/VA) is also reduced, it is suggestive of parenchymal or pulmonary vascular disease.
Lung Volumes
Measurement of absolute lung volumes, including RV, FRC, and TLC (Figure 2), is more challenging, and their use is limited in clinical practice. However, they are sometimes strictly necessary for a correct diagnosis. The definition of restrictive lung disease is based on reduced total lung capacity (TLC), which is the amount of air in the lung after maximum inspiration. The amount of air in the lungs after maximum expiration is residual volume (RV). In a patient with reduced FVC (or VC) on spirometry, measurement of lung volumes is necessary to exclude or confirm restriction. A normal TLC rules out true restriction. Increased TLC in a patient with reduced FER (obstruction) and reduced FVC confirms hyperinflation related to severe airflow obstruction. Reduced TLC in a patient with reduced FVC and normal FER (restriction) confirms the diagnosis of true restriction. Reduced TLC in a patient with reduced FER (obstruction) and reduced FVC confirms a mixed obstructive-restrictive defect. These findings are summarized in Table 5.
Measurement of RV is informative in some patients. An increased RV/TLC ratio more than predicted in a patient with an obstructive ventilatory defect is indicative of gas trapping. In a patient with neuromuscular disease with a restrictive ventilatory defect, reduced TLC is typically associated with increased RV.
Bronchial Challenge Tests
There are two types of bronchial challenge tests:
1. **Indirect challenges**: Activate mast cells to release histamine and other bronchoconstrictor mediators (e.g., mannitol, hypertonic saline, eucapnic voluntary hyperventilation, exercise challenge).
2. **Direct challenges**: Directly constrict airway smooth muscle via receptors on smooth muscle (e.g., methacholine, histamine).
These tests are considered positive if a known dose of stimuli can cause a 20% (direct challenge) or 15% (indirect challenge) reduction in FEV1. These tests are useful in excluding, rather than confirming, a diagnosis of asthma.
There are 2 components of airway hyper-responsiveness: inflammation and persistent airway remodeling. A greater change in responsiveness to indirect stimuli (e.g., mannitol, hypertonic saline) is suggestive of a more inflammatory component and the need for treatment with inhaled corticosteroids. On the other hand, a greater response to direct airway stimuli (methacholine, histamine) is suggestive of persistent airway remodeling. Response to direct stimuli may decrease during treatment with ICS but does not resolve.
A positive indirect test is consistent with a diagnosis of active asthma (high specificity) and predicts a response to treatment with inhaled steroids. They are also used in diagnosing exercise-induced bronchoconstriction (EIB) and identifying individuals who may experience “airway narrowing” while SCUBA diving.
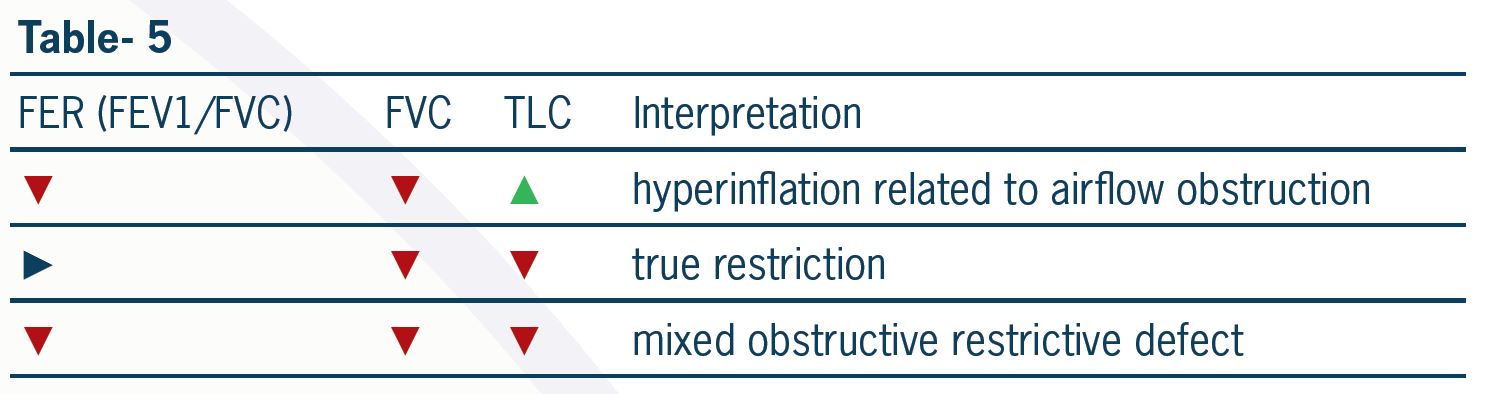
Table 6 shows some clinical advantages of the mannitol challenge test in the diagnosis and management of patients with respiratory disorders.
In an asthmatic patient with normal lung function and a negative indirect challenge test, a regular long-acting β2-agonist is probably not warranted.
Patient Follow-Up and Repeat RFTs to Assess
Progression of Disease
One of the main indications for respiratory function tests is in following up patients and screening for the progression of chronic lung diseases.
In obstructive lung disease, a decline in FEV1 by more than 12% and 200 ml is considered significant. As a general rule, a decline in DLCO by more than 3 units is considered significant.
A positive bronchodilator response or a positive bronchial challenge test in an asthmatic suggests current airway hyper-responsiveness and the need for further therapy.
In idiopathic pulmonary fibrosis, FVC and TLC correlate poorly with the morphologic extent of disease on HRCT and are less predictive of outcome than DLCO. A 10% change in FVC or 15% change in TLCO is considered significant in the management of interstitial lung disease.
DLCO is a good parameter in the follow-up of patients with combined emphysema and interstitial lung disease and ILD combined with pulmonary hypertension.
About Us
At RSDC we have a particular interest in quick approach and triaging patients with suspected lung malignancy, management of pleural diseases, sleep disorders of obstructive sleep apnoea and other more complex sleep disorders, airways disease including asthma and COPD.
We use a comprehensive approach to interstitial lung disease in addition to occupational and environmental lung disease.