Pleural diseases and investigations (RACP lecture)
Introduction
- The pleural space contains 10-25 millilitres of fluid
- Created in the pleural capillaries and lymphatics, largely reabsorbed via the lymphatics of the parietal pleura
- > 3000 /million population develop pleural effusion each year
- There are 60 different causes of pleural effusions
- Left ventricular failure is the most common cause
- Delay in diagnosis and management contribute to morbidity
- The number of pleural procedures available to physicians israpidly expanding
- Pleural services that provide high-quality and promptintervention will improve patient care
Pathogenesis
- Excess pleural fluid formation or decreased fluid reabsorption
- Increased pulmonary capillary pressure (e.g. heart failure)
- Increased pulmonary capillary permeability (e.g. pneumonia)
- Decreased intrapleural pressure (e.g. atelectasis)
- Decreased plasma oncotic pressure (e.g. hypoalbuminaemia)
- Obstruction of pleural lymphatic drainage (e.g. malignancy)
- Fluid from other cavities or sites (e.g. peritoneal,retroperitoneal fluid)
- Rupture of thoracic vessels (e.g. haemothorax, chylothorax)
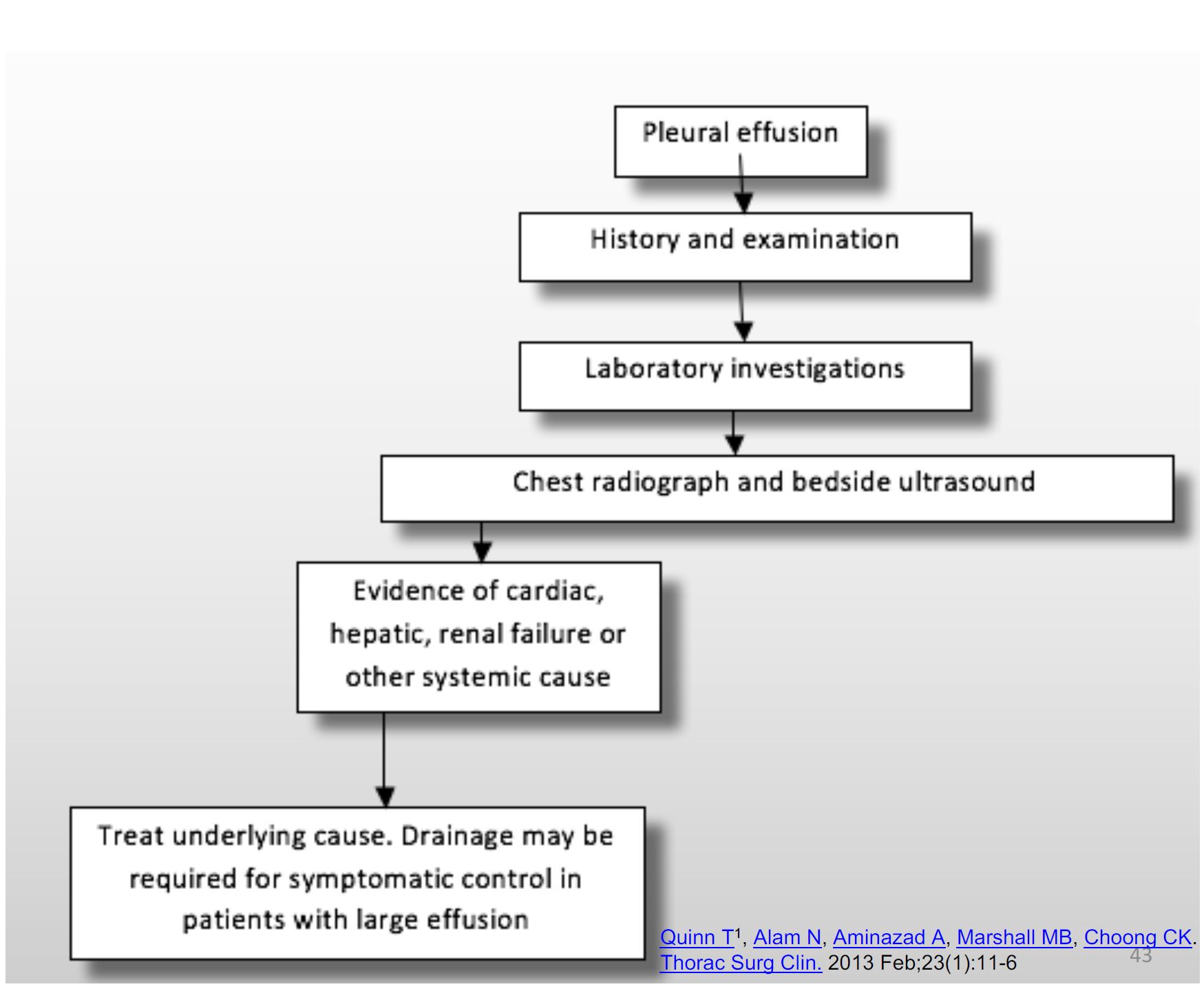
Approach to patient
History and Examination
- The history is targeted to find the cause of the effusion
- Clinical examination be performed with causes of effusions in mind. Unilateral or bilateral, further aiding diagnosis
Investigations
Blood tests
- Full blood count including white cell differentiation (cansuggest infection or haematological malignancy)
- Biochemistry (e.g. renal impairment)
- Liver function tests (hepatic causes)
- Albumin
- Lipase
- LDH
- Blood cultures and serology
- Investigations for autoimmune conditions
Radiological tests
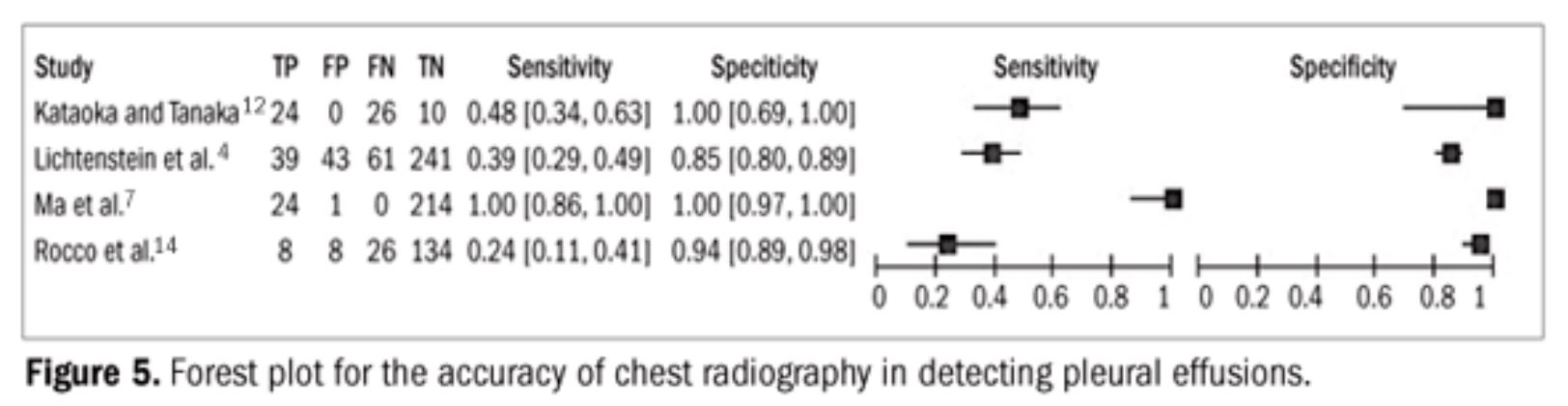
- CXR: Sensitivity and specificity of 65% and 81% respectively incritically ill patients compared with bedside ultrasound
- Ultrasound – accuracy and convenience, locating small effusions, areas of loculation and guiding thoracocentesis
- Bedside ultrasound is becoming a routine part of pleural effusion management
- CT– further identify the causes of the effusion, size, location and presence of loculations
- PET– may be useful in malignant effusions
Pleural Ultrasound
- Identify pleural effusions and abnormalities
- Differentiate effusion from thickening or consolidation
- Best site to aspirate in small or multiloculated effusion
- Real-time guided pleural aspiration or biopsy
- Sensitivity 73% Specificity 100%
Pleural Ultrasound Clinical Indications
- Physical exam has low discriminatory value between effusion and solid organs below diaphragm
- Accuracy of clinical examination for effusion PPV 86% NPV 46% Sensitivity 77% Specificity 60%
- Clinical exam failed to or wrongly identified sites in 32%
- No difference ‘consultants’ vs ‘senior reg’ vs ‘juniors’
US vs CXR
Meta-analysis of 4 studies
US
- Sensitivity 93%
- Specificity was 96%
CXR
- Sensitivity 24-100%
- Specificity 85-100%
X Marks the spot
- Real time needle puncture or immediate preprocedure marking
- Marking and puncture at later time or location doesn’t reduce iatrogenic pneumothoraces
Thoracocentesis
- The pleural fluid should always be sampled unless the underlying cause is known (for example heart failure). Best to be done under ultrasound guidance.
- The macroscopic appearance of the fluid should be analysed – milky white (chylothorax), turbid (empyema), bloodstained (trauma, malignant, parapneumonic), food particles (oesophageal rupture)
Send for the right test
- Pleural fluid for: protein, LDH, cholestrol, MCS, cytology, AFB
- Differentiation of cell counts
- Serum for: LDH, protein
- Suspect infection: pH, glucose, TB-PCR, ADA
- Suspect chylothorax: triglycerides, chylomicron
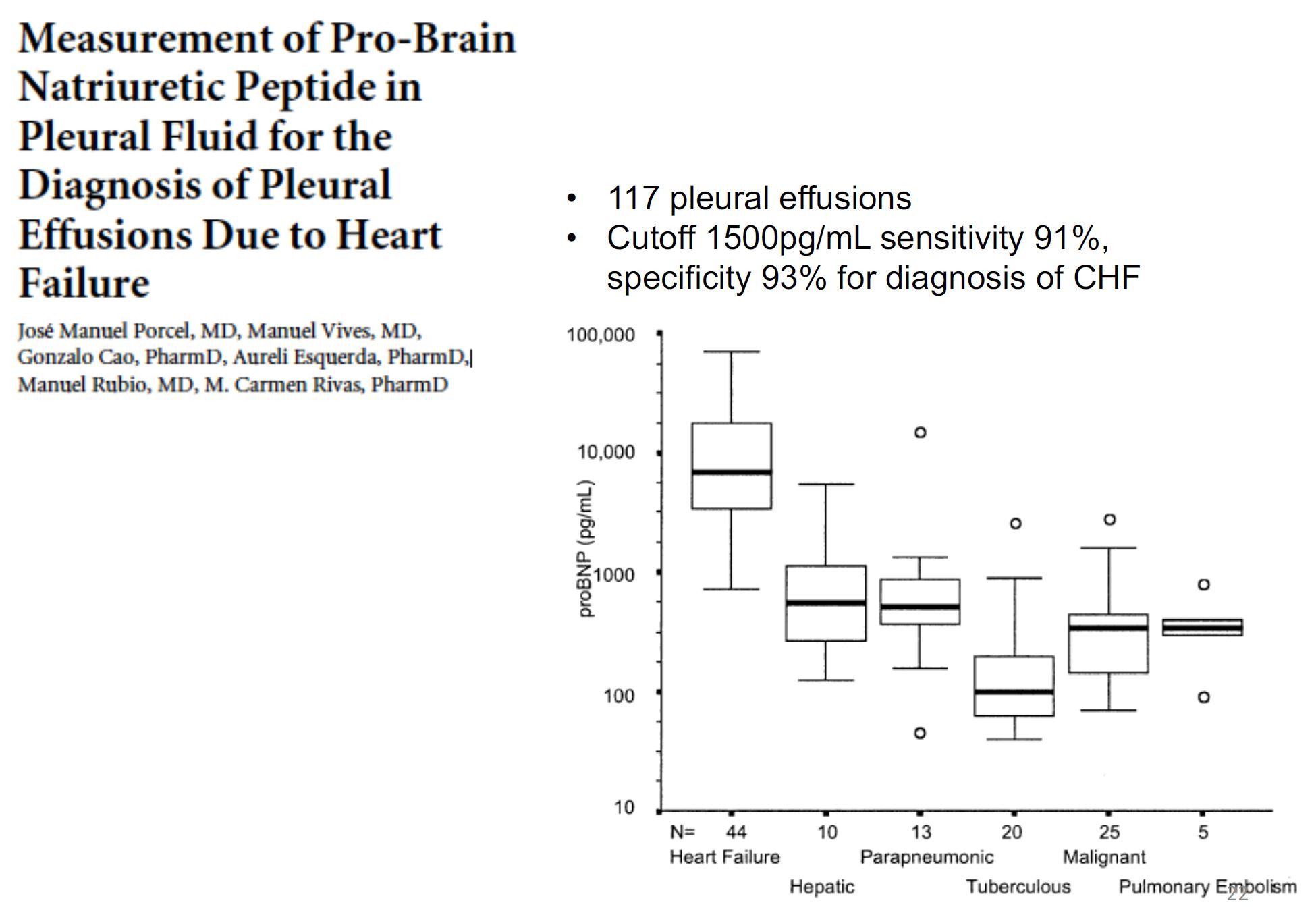
- Other test to consider: Albumin, Pro-BNP, RF, Amylase, Haematocrit
Light’s criteria
- Pleural fluid is exudate if ONE or more of the following criteria are met
- Pleural fluid protein / serum protein >0.5
- Pleural fluid LDH / serum LDH >0.6
- Pleural fluid LDH >2/3 upper limit of laboratory normal value for serum LDH
- Light’s criteria has a diagnostic accuracy of 93-96%
- Caution FALSE exudate in CCF + Diuresis
Two Test and Three Test Rules
Two test rule
- Pleural fluid cholesterol >45mg/dL (1.165 mmol/L)
- Pleural fluid LDH >45% upper limit of normal LDH
Sensitivity 97.5%, specificity 71.9%
Three Test Rule
- Pleural fluid protein >29 g/L
- Pleural fluid cholesterol >45 mg/dL (1.165 mmol/L)
- Pleural fluid LDH greater than 45% upper limit of normal lab LDH
Sensitivity 98.4%, specificity 70.4%
pH is also valuble
- pH is an important laboratory test in management of parapneumonic effusions.
- In patients with parapneumonic effusion if PH<7.2 à complicated parapneumonic effusion
- Necessitates fluid drainage by intercostal catheter insertion or surgery
Inclusion of air -> increases pH
Local anaesthetic -> lowers pH
DDX of transudate
Exudate (due to increased capillary permeability)
- Infection (most common cause)
Bacterial, Tuberculosis, Fungal, Viral, Parasitic
- Neoplastic
Primary lung cancer, Metastatic disease to pleura, Mesothelioma, Lymphoma, Paraproteinemia
- Infradiaphragmatic
Pancreatitis, Peritonitis , Bile duct obstruction, Intraabdominal abscess, Meig’s syndrome (ascities and pleural effusion associated with pelvic tumours)
Causes- Exudate (Cont.)
- Immune conditions
CTD, vasculitis/granulomatous diseases, Sarcoidosis (rare)
- Medications
Amiodarone, Bromocriptine, Dantrolene, Metronidazole Nitrofurantoin, Procarbazine
- Post operative / Iatrogenic
Post cardiac surgery, Lung transplantation (and also rejection), Abdominal surgery
- Other
Haemothorax, Chylothorax, Benign asbestos related effusion,Oesophageal rupture,Yellow nail syndrome ,Amyloidosis
False Exudates
- Current criteria can falsely classifies transudates as exudates
- Most falsely classified as exudate by only 1 of 3 elements of Light criteria
- Due to diuretics
- Albumin gradient >1.2g/dL and protein gradient >3.1g/dL used to reclassify exudate to transudates
Ddx of low pleural fluid glucose
Low is defined as <3.4mmol/L
- Complicated parapneumonic effusions/Empyema
- Rheumatoid arthritis
- TB
- Malignancy
- Esophageal rupture
Cell differential may help
- Neutrophils – acute process
- Lymphocytes – chronic process/long standing effusions
>80% think TB, lymphoma, rheumatoid, sarcoidosis
- Eosinophils (>10%)
Most common cause is air or blood in the pleural space
Management
Benign pleural effusion
• Management will depend on the cause of the effusion.ood in the pleural space
Symptomatic relief and diagnosis can be achieved with drainage
• Chest drain insertion (seldinger technique/ catheter over needle)
Risks
Bleeding
• Neurovascular bundle
• Greater variability elderly and posteriorly (aim 9-10cm laterally to spine)
Infection
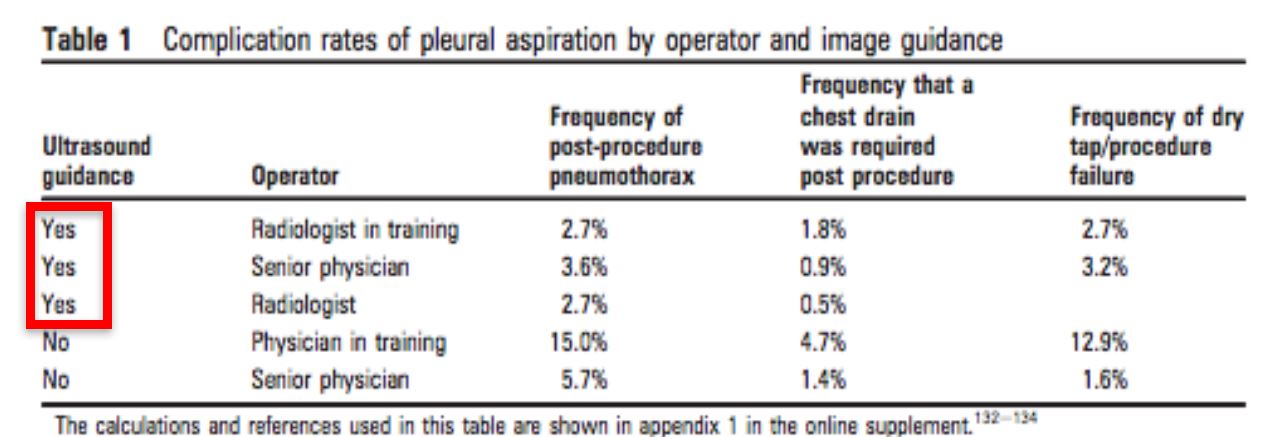
Pneumothorax
- US Guided 1.3-6.7%
- Without US 4-30%
Re-expansion pulmonary oedema
- Large volume of fluid removed
- Rare 0.2%-0.5%
- Negated by limiting drainage 1- 1.5L
- Limit end expiratory pressure < – 20 cmH2O. Reduces chest discomfort and reduces cough
Tube size
- BTS recommendation: Small drains should be used as first-line therapy for pneumothorax, free flowing pleural effusions, pleural infection and pleurodesis (C)
- Seldingers good in the obese
Chest Tubes
- Traditionally large bore (≥ 28F) recommended
- Recent years smaller tubes ≤ 15F more common
- Advantages of smaller tubes
Easier insertion
Less pain during and whilst inserted
Lower risk of complications
Volume of removal in single aspirate
- Procedure should be stopped when no more fluid or air can be aspirated, the patient develops symptoms of cough and chest discomfort, or has 1.5L drained
BTS
- Ultrasound guidance should be mandatory
- Bedside Ultrasound NOT x-mark the spot
- Training is important
- Limit use to trained persons
- Pleural team
- Recognize limitations; work with radiologists / sonographers
Malignant effusions
- Patients should be discussed within a multidisciplinary team to determine the best management
- Malignant pleural effusions can be drained for symptom relief
- Many of these effusions are likely to recur
- Surgical or bedside pleurodesis or indwelling pleural catheter (IPC) insertion is recommended depending on life expectancy 1
- Repeat thoracocentesis in some patients
Patient Prognosis
- Depends on histologic type ,chemotherapy-responsive
- mesothelioma > metastatic adenocarcinoma
- Performance Score significant predictor
- KPS score ≥70: median survival 395 days
- KPS score ≤ 30: 34 days
- Pleural pH ≤ 7.28: poorer prognosis(weak)
- Pleural Mesothelioma-parietal pleura first affectedprognosis is better if only parietal (not visceral) pleura affected (33 vs 7 months)
Thoracoscopy / Pleuroscopy
- Performed under general anaesthesia but can be done using local anaesthetic and sedation.
- Direct visualisation of the pleural, biopsy and pleurodesis can be simultaneously performed.
- Thoracotomy may be performed if other methods have failed.
Pleurodesis
- Pleurodesis is preferable to recurrent thoracocentesis in management of malignant effusions
- Pleurodesis can be done by introducing a talc slurry through an intercostal catheter
- Alternatively, it can be performed with aerosolised talc which can be used during thoracoscopy
- The advantage of thoracoscopy is that loculations or adhesions can be visualised and divided, aiding in lung reexpansion. Also aerosolised talc can be distributed throughout the pleural cavity under vision
Pleurodesis: Patient Selection
- Symptomatic and Recurrent pleural effusions
- Symptom relief with fluid drainage
- How recurrent?
- Probably higher success rate with early pleurodesis
- Patient with ‘reasonable’ life expectancy
- > 3 months (arbitrary)
- Performance Status important
Pleurodesis –with what?
- Talc-Tetracycline/Doxycycline-Bleomycin-OK432 / Staphylococcal Enterotoxin B-Quinacrine
- Evolving Concept: TGFs2
- Potent cytokine in stimulating extracellular matrix synthesis
- Induces mesothelial cell production of collagen
Talc and hypoxemia
- Talc induces systemic and lung inflammation, and worsens hypoxia –regardless as slurry (Maskell et al)or poudrage (Janssen et al)
Thoracoscopic (large) talc poudrage in >550 patients
- No patients had ARDS
- Poudrage still followed by significant O2 desaturation
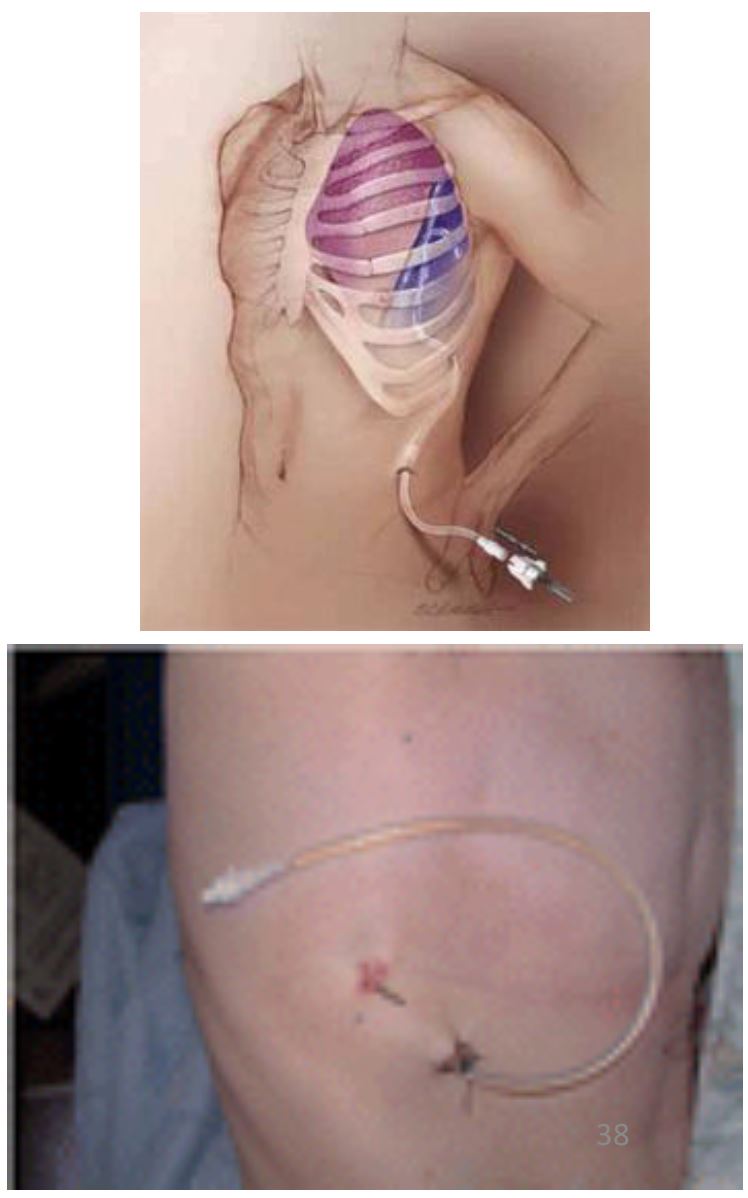
Tunnelled Indwelling Pleural Catheter
- Indications: Failed Pleurodesis; Trapped Lung
Advantages:
- Management of pleural effusion out of hospital
- Patients with terminal illness to spend time at home
- Reduce hospital costs
- Fewer hospital bed days, fewer pleural procedures, no difference in infection rates compared with pleurodesis
- Spontaneous pleurodesis in up to 70%
Parapneumonic effusions and empyema
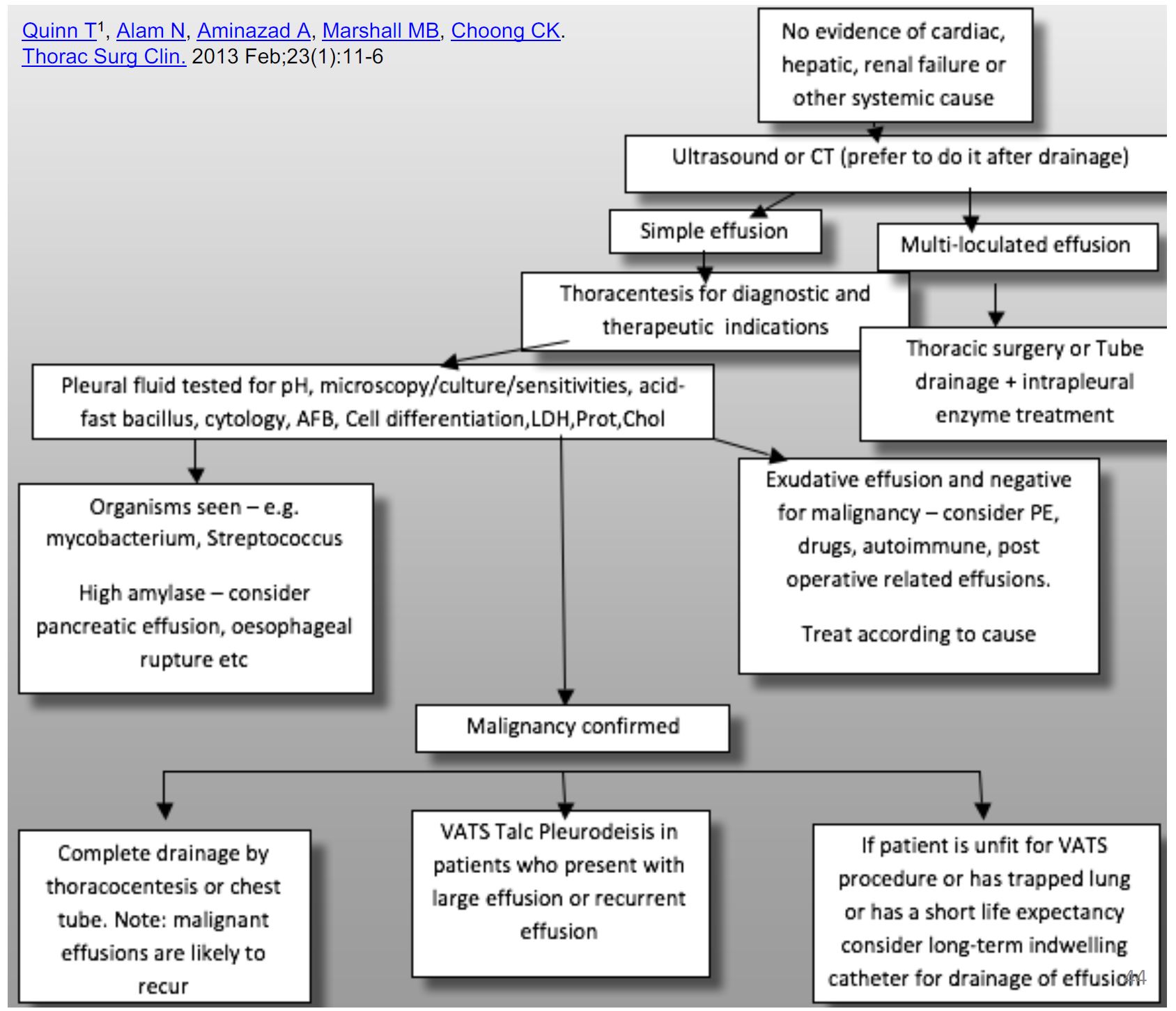
- It is crucial to differentiate between simple parapneumonic effusions and complex parapneumonic effusions or empyema as the latter needs drainage with thoracentesis or thoracic surgery.
- Empyema: frankly purulent fluid or positive Gram stain/culture.
- Complicated parapneumonic effusion:presence of loculations (found by thoracic ultrasound) or pH<7.2
Complicated para-pneumonic effusions
- May not respond to thoracentesis alone.
- Intrapleural enzyme treatments to break the locules and improve pleural fluid drainage.
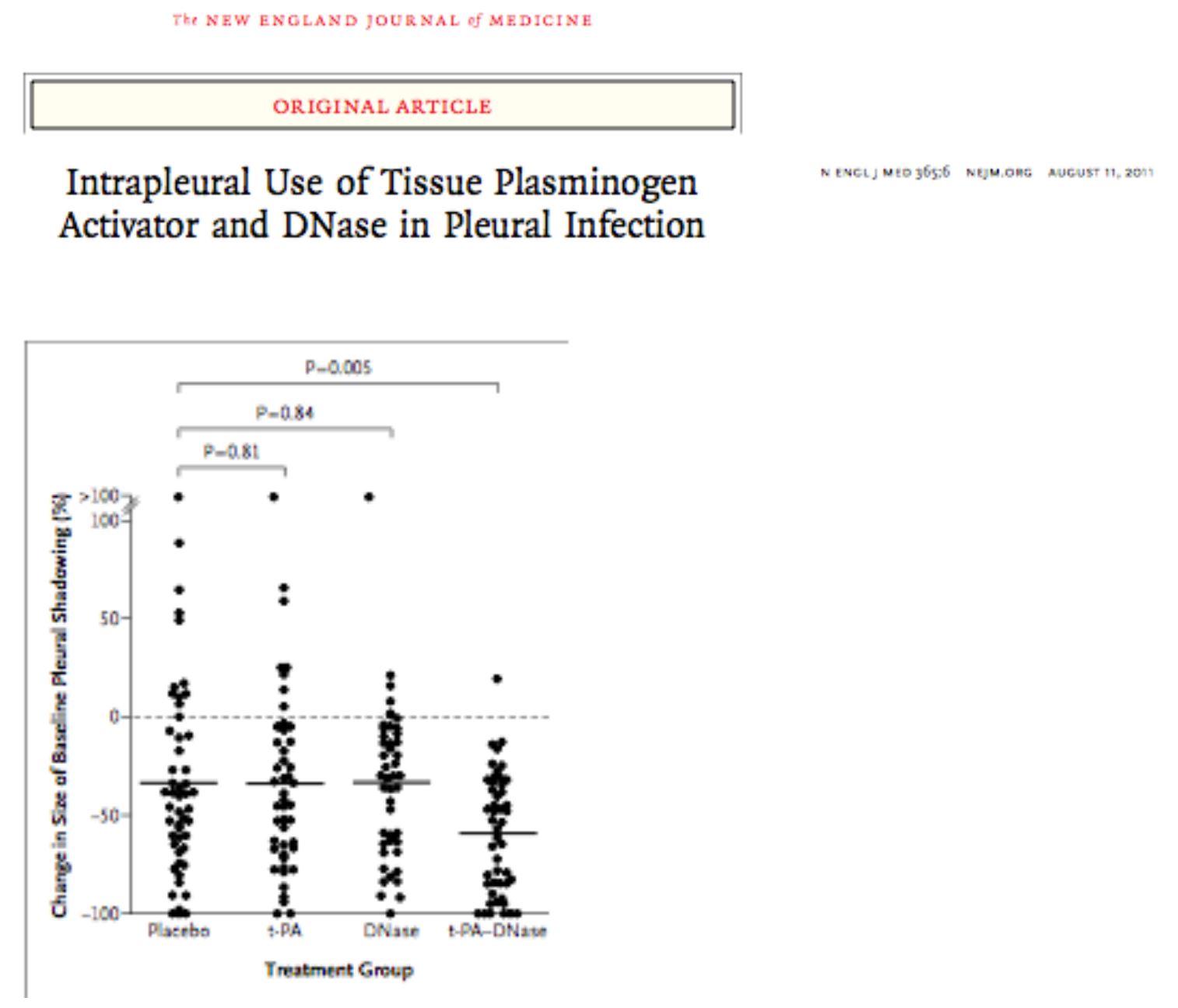
- Blinded randomised controlled trial, intrapleural t-PA and Dornase compared with either of these treatments alone improved fluid drainage, reduced frequency of surgical referral and duration of hospital stay.
- Thoracic surgery remains a treatment modality in the management of complicated pleural effusions
Persistent undiagnosed effusion
- A significant number of patients with pleural exudates are diagnosed with non-specific pleurisy
- Retrospective study of 75 patients
8% turn out to be malignant over 2 years
92% followed a benign course with spontaneous resolution in 82% of cases
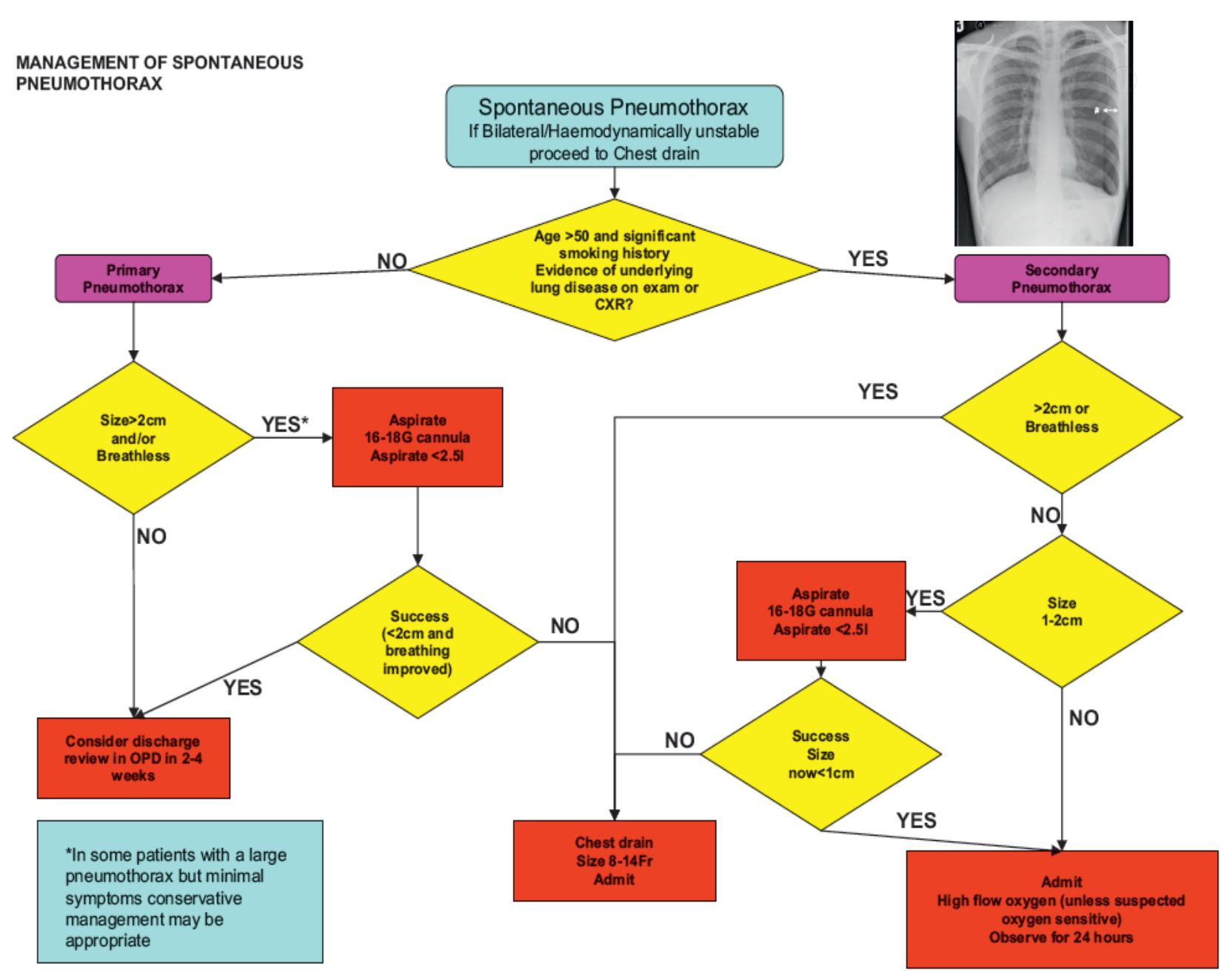
Spontaneous Pneumothorax
- Primary spontaneous pneumothorax (PSP) àno underlying lung disease
- Secondary spontaneous pneumothorax (SSP) àpresence of underlying lung disease
Needle aspiration or chest drain
- Needle aspiration should only be done ONCE
- Following failed needle aspiration, a chest drain insertion is recommended
- 14-16G aspiration is as effective as large bore >20F chest drains
- Guidelines that encourage needle aspiration are not always followed and the ease of insertion of small bore <14F chest drains may be regarded as a simpler option
Thoracic Surgery?
- Persistent air leak or failure of the lung to re-expand, an early (3-5 day) thoracic surgical opinion should be sought
- Open thoracotomy and pleurectomy remain the procedure with the lowest recurrence rate (~1%) for difficult or recurrent pneumothoraces
- VATS with pleurectomy and pleural abrasion is better tolerated but have a higher recurrence rate of ~5%
Management of SSP
- All patients with SSP should be admitted for at least 24hrs + receive oxygen if possible
- Most will require the insertion of a small bore chest drain
- Those with persistent air leak should be discussed with a thoracic surgeon at 48hrs
- Medical pleurodesis may be appropriate for patients not suitable for surgery
- An alternative is ambulatory management with a Heimlich valve
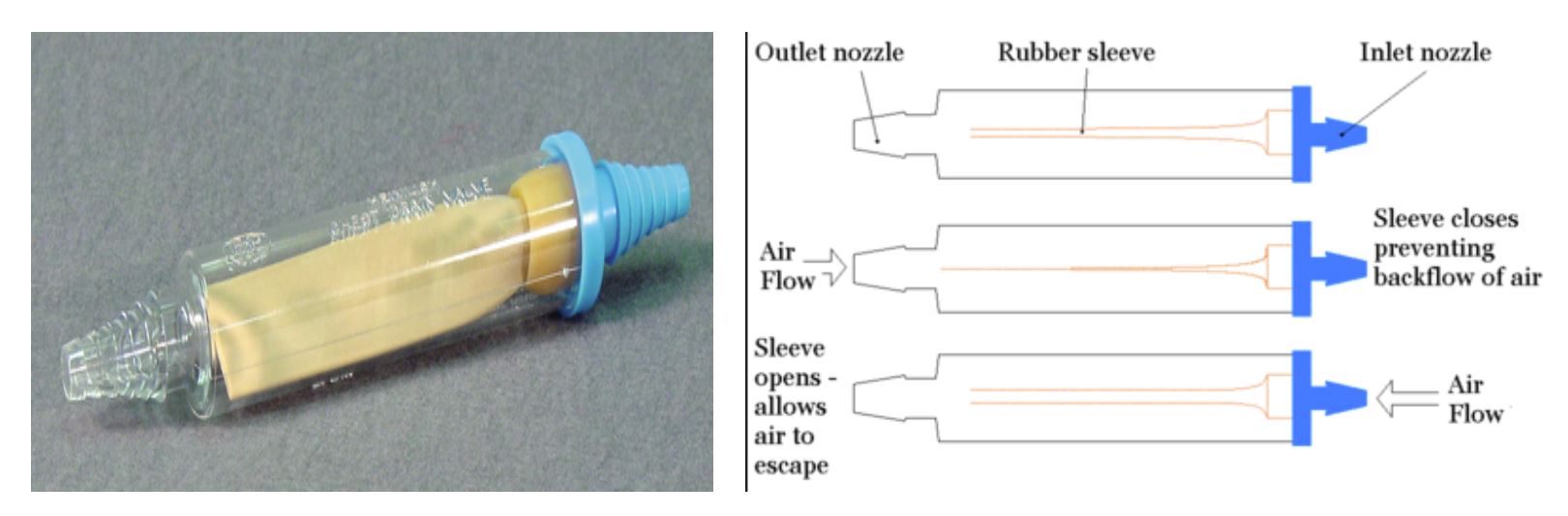
Heimlich Valve
Discharge and follow-up post Pneumothorax
A significant number of patients with pleural exudates are diagnosed with non-specific pleurisy
- Retrospective study of 75 patients
8% turn out to be malignant over 2 years
92% followed a benign course with spontaneous resolution in 82% of cases
Persistent undiagnosed effusion
- Air travel should be avoided until full resolution (Guideline at least 2-4 weeks)
- Diving should be permanently avoided unless the patient has undergone bilateral surgical pleurectomy and has normal lung function and normal post-op chest CT scan
- Those managed by observation alone or by needle aspiration should be advised to return for a follow-up CXR after 2-4 weeks to monitor resolution
- There is NO evidence to link recurrence of pneumothorax with physical exertion